|
|
|
Research
Steps of research, essential elements, and overall approach in PAAR4Kids.
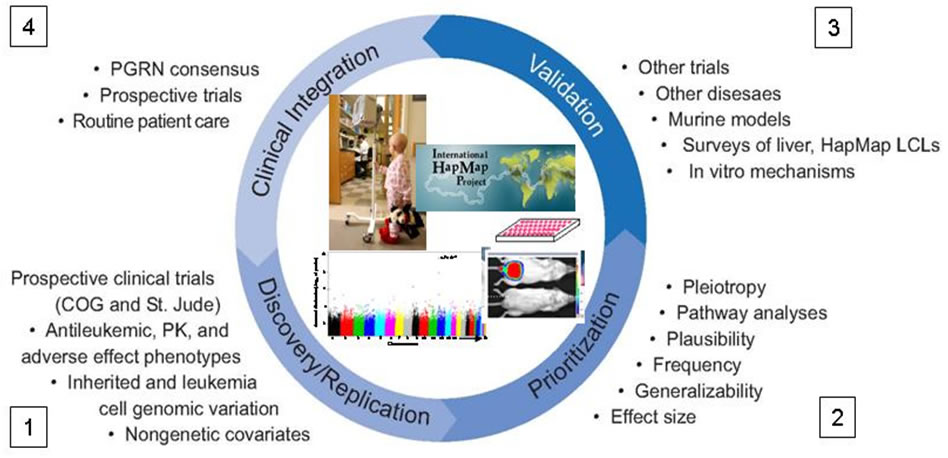
Step 1: Discovery/replication: The core prospective clinical trials of COG and St. Jude serve as the primary discovery and replication cohorts for PAAR4Kids. Detailed phenotypes, genome-wide and candidate genotyping in germline and in ALL tumor DNA, and nongenetic covariates affecting phenotypes are analyzed in genotype/phenotype association studies.
Step 2: Prioritization: a number of criteria (e.g. pleiotropy, biological pathways, MAF, effect size, conservation) are used to prioritize which associations are most important to further confirm.
Step 3: Validation: can entail additional genotype/phenotype association analyses in other “non-core” ALL trials (e.g. European collaborators, COG AALL03N1, other PGRN trials, NHANES, etc) and can encompass surveys of other human tissues (e.g. human liver resource, HapMap LCLs) and mechanistic follow-up studies (e.g. in murine models or in vitro tools such as knock-down, directed expression).
Step 4: Clinical integration: For those genotype/phenotype associations with large effect sizes that have been solidly validated, whether they emanate from our own work or that of others, we will work to incorporate pharmacogenomic-based drug dosing into clinical trials or routine patient care. After treatment is modified based on polymorphisms with the greatest effect, then other polymorphisms may emerge in future discovery/replication studies (back to Step 1)--hence the circle.
Medications used to treat childhood ALL
Prednisone/Dexamethasone
Asparaginase
Vincristine
6-mercaptopurine/thioguanine
Methotrexate
Daunorubicin
Cyclophosphamide
Cytarabine
Etoposide/clofarabine/imatinib/dasatinib
Core and Non-Core Clinical Trials
Core and non-core clinical trials that are primary discovery/replication sources for PAAR4Kids.
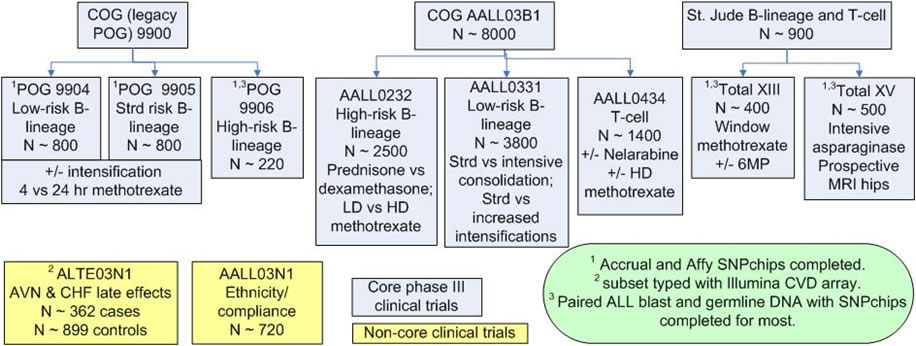
Prospective clinical trials included in PAAR4Kids
| Parent protocol* |
Therapeutic trial Mnemonic# (sample sizes) |
PI |
Therapeutic trial title |
Examples of evaluable phenotypes |
COG (legacy POG) 9900 |
POG 9904/9905 (n~1600) |
Martin, Winick |
ALinC 17 Treatment for Patients with Low Risk ALL (9904) or Standard Risk ALL (9905) Phase III Studies
|
MRD, relapse, VCR neuropathy
|
POG 9906 (n~220) |
Bowman |
ALinC 17: Protocol for Patients with Newly Diagnosed High Risk ALL - Evaluation of the Augmented BFM Regimen: A Phase III Study
|
MRD, relapse, VCR neuropathy, ON, asparaginase allergy, pancreatitis, blast gene expression
|
COG
AALL03B1; PI: Loh, Raetz
AALL08B2; PI: Loh, Relling |
AALL0232 (n~2500) |
Larsen |
High Risk B-precursor ALL
|
MRD, relapse, ON, pancreatitis, asparaginase allergy, VCR neuropathy
|
AALL0434 (n~1400) |
Winter, Dunsmore |
Intensified MTX, Nelarabine and Augmented BFM Therapy for Children and Young Adults with Newly Diagnosed T-cell ALL
|
MRD, relapse, MTX neurotoxicity, pancreatitis, asparaginase allergy, VCR neuropathy
|
AALL0331 (n~3800) |
Maloney |
Standard Risk B-precursor ALL
|
MRD, relapse, pancreatitis, asparaginase allergy, VCR neuropathy
|
| NA |
COG AALL03N1 (n~720) |
Bhatia |
Understanding the Ethnic and Racial Differences in Survival in Children with ALL--not a front-line core trial
|
Medication compliance, relapse, TPMT activity, erythrocyte thiopurine measures
|
| NA |
COG ALTE03N1 (n~1261) |
Bhatia |
Key Adverse Events After Childhood Cancer--not a front-line core trial--case control
|
Anthracycline cardiotoxicity, ON and controls
|
| NA |
St. Jude Total XIII (n~400) |
Pui |
Total Therapy Study XIIIA & B for Newly Diagnosed Patients with ALL |
MRD, relapse, ON, pancreatitis, asparaginase allergy, VCR neuropathy, height, weight, PK of MTX, thiopurine and etoposide, in vitro ALL drug sensitivity, blast gene expression
|
| NA |
St. Jude Total XV (n~500) |
Pui |
Total Therapy Study XV for Newly Diagnosed Patients with ALL |
MRD, relapse, ON, pancreatitis, asparaginase allergy, VCR neuropathy, blast gene expression, height, weight, cortisol, lipids, PK of MTX, thiopurine, and dexamethasone, in vitro ALL drug sensitivity
|
| * The parent protocols describe biology, induction therapy, and genetic studies but the primary patient enrollments, continuation therapy, and phenotypes are determined in patients enrolled on the therapeutic trials listed under trials column#. VCR=vincristine; ON=osteonecrosis; NA=not applicable. Numbers indicate those with DNA. |
|
|